The evolution of PQA’s continued involvement in medication therapy management (MTM) spans over a decade. Milestones include endorsement of the Completion Rate for Comprehensive Medication Review performance measure in 2011 and development of the foundational Medication Therapy Problem Categories Framework in 2017.
In 2023, PQA launched a multiphase national MTM initiative to address stakeholder needs and advance MTM quality measurement. A draft call to action was created using findings from an environmental scan and a PQA Convenes event. Then, in August 2024, PQA released the Advancing Medication Therapy Management Quality Measurement report informed by public comments solicited from PQA members and MTM stakeholders. The report issued an eight-point call to action with elements needed to advance the quality of MTM services within the Medicare Part D program.
PQA launched the MTM Advisory Group in September 2024 to gain stakeholder feedback, consensus, and momentum to address short- and long-term objectives outlined in the report to advance MTM service quality and measurement.
As a first step, the PQA Blueprint 2025 was leveraged to shape efforts, and objectives, strategies, and tactics were developed to strategically support success of the MTM initiative. The goal of the MTM Advisory Group is to identify opportunities to improve the quality of medication management services, focused on Medicare Part D MTM program goals and services. The activities will be within the scope of PQA’s mission, strategic plan, expertise, and influence.
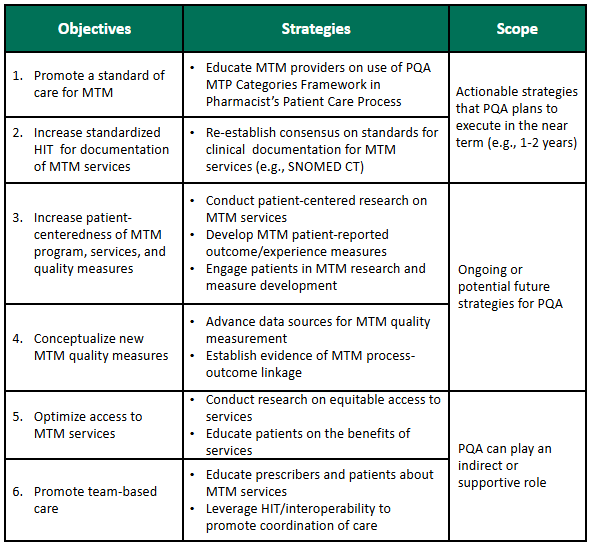
PQA has identified six objectives that can advance MTM quality and measurement, and the MTM Advisory Group is serving as a forum providing expert feedback on how to approach these.
There are two objectives that PQA will work to address in the next two years. PQA will promote a standard of care for MTM by educating MTM providers on use of the PQA Medication Therapy Problem (MTP) Categories Framework as part of the Pharmacists’ Patient Care Process. PQA also will support increased use of standardized health information technology (HIT) for MTM services by re-establishing consensus on standards for clinical documentation of MTM services related to medication therapy problem recommendations and resolution using Systematized Nomenclature of Medicine - Clinical Terms (SNOMED CT) codes.
Two additional objectives are ongoing or future priorities for PQA:
- to increase the patient-centeredness of MTM programs, services, and quality measures through research, and
- to conceptualize new MTM quality measures.
Part of this effort includes PQA’s previous research to better understand patients’ experiences with comprehensive medication reviews, which led to the creation of a framework that can be used for continued quality improvement.
The last two objectives are areas where PQA can play a supportive role, but broader industry effort is needed to optimize access to MTM services and promote team-based care.
Additional detail on these objectives is provided in the table below.
Of note, quality measure development for MTM is beyond the scope of the MTM Advisory Group, and would occur through PQA’s systematic, consensus-based measure development process.
The MTM Advisory Group is slated to convene through August 2025 and will periodically assess progress towards prioritized goals and objectives, to evaluate the value of continued meetings. Participation in the group is open to any individual at a PQA member organization. Individuals interested in joining the group should complete the group participation request form.
