Piloting the PQA Specialty Pharmacy Turnaround Time Quality Measure
The use of specialty medicine has grown rapidly in recent decades, fueling parallel growth in the number of specialty pharmacies that serve patients who are prescribed specialty medications. Patients receiving specialty medications often have complex, high-cost treatment needs, necessitating close management from care teams, including specialty pharmacies. Specialty pharmacies dispense medications, for which timely initiation of therapy is a clinical priority to optimize outcomes.
Recognizing this, specialty pharmacy stakeholders have articulated the need for standardized turnaround time (TAT) measurement and reporting to better understand a key aspect of specialty pharmacy quality. There are known barriers and facilitators that can influence TAT across different specialty pharmacies, and measurement is a critical tool to evaluate quality and drive improvement.
Background: PQA’s Efforts to Standardize TAT
In 2017 and in collaboration with NASP, PQA identified and prioritized specialty pharmacy measure concepts for development. These concepts were then further evaluated during a workshop with specialty pharmacy stakeholders, during which turnaround time emerged as a high priority for measure development. From 2018-2019, PQA convened a multistakeholder Specialty Pharmacy Turnaround Time Task Force to develop specifications for the measure concept, focused on establishing a standardized measure of the time between a specialty pharmacy receiving a new prescription for a specialty medication and the product being available for the patient (i.e., ready for pick up or scheduled for delivery). The measure concept continued through PQA’s multistakeholder, consensus-based development process, and the Specialty Pharmacy Turnaround Time [Pharmacy] (SP-TAT-PH) pharmacy quality measure was endorsed through a PQA membership vote in 2021.
In parallel with measure development, PQA initiated a research project in 2020 to identify facilitators and barriers impacting specialty pharmacy prescription turnaround time. Results of this work were published in the Journal of Managed Care & Specialty Pharmacy in October 2022.
Pilot Design: Using The SP-TAT-PH Measure in Real World Settings
In 2022, with funding support from Pfizer, PQA launched the PQA Specialty Pharmacy Turnaround Time Implementation Pilot to advance standardized measurement of TAT. Project aims included implementing the PQA-endorsed SP-TAT-PH measure to better understand the data sources used to calculate the measure, evaluate potential refinements to measure specifications, identify opportunities for improvement, and add to the collection of barriers, facilitators and promising practices for optimizing TAT. Five organizations—across the chain, independent, integrated delivery network (IDN) and PBM-owned specialty pharmacy models—participated in the pilot, and included Walgreens, Noble Health Services, Kaiser Permanente, Vanderbilt Specialty Pharmacy, and Accredo by Evernorth.
Pilot participants were provided a technical package containing all resources needed to accurately calculate the SP-TAT-PH measure. Measure rates were calculated at baseline (October 2021 – September 2022), midpoint (October 2022 – March 2023) and endpoint (October 2022 – September 2023) for each specialty pharmacy organization’s participating pharmacies.
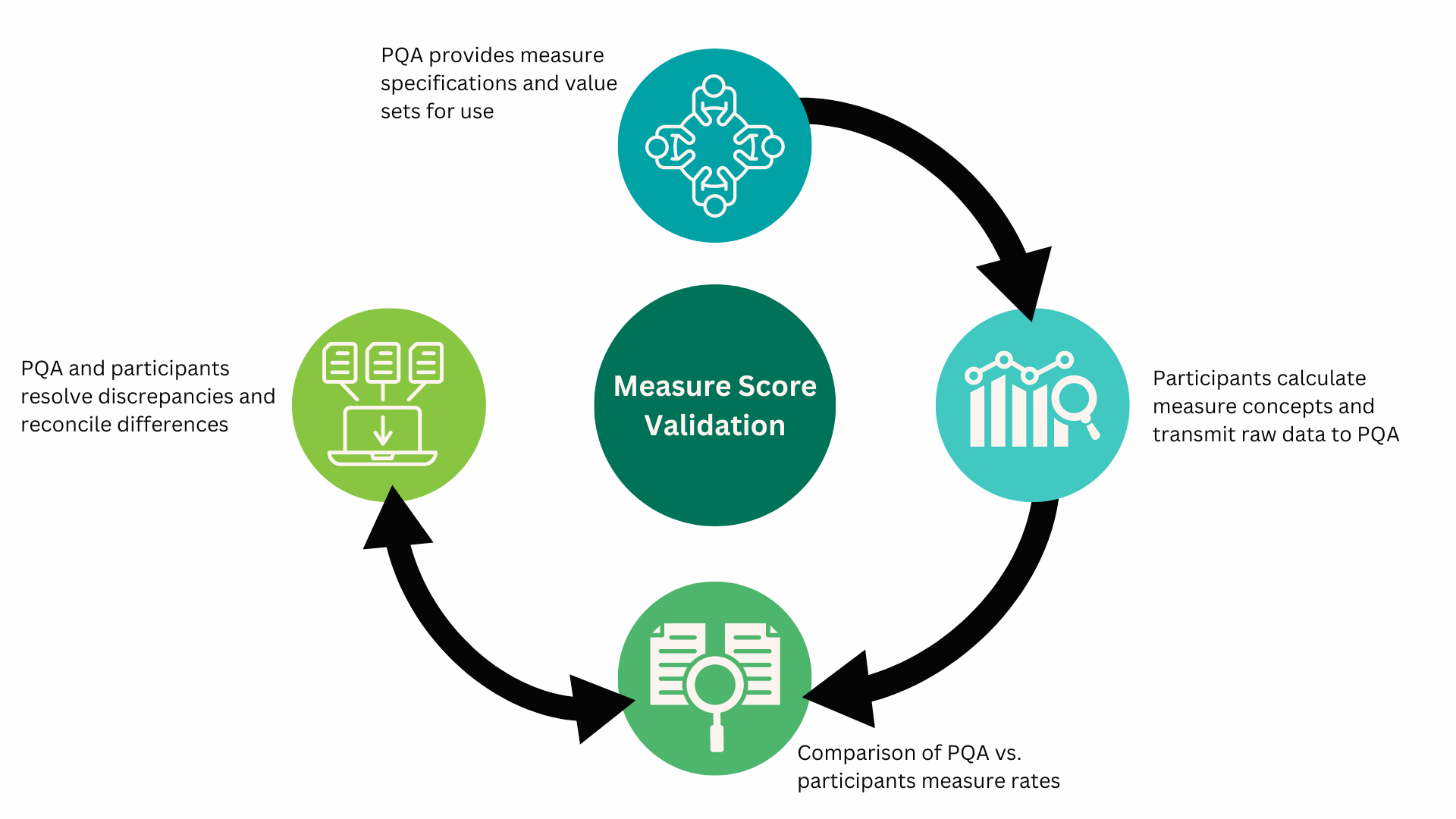
PQA received raw data from participants to independently validate results – a critical step in the process to ensure accurate measure calculations. Wherever discrepancies between PQA’s and participants’ results were identified, PQA and participants worked iteratively to understand and resolve the differences. Figure 1, illustrates the measure validation process.
Figure 1. PQA Pilot Measure Validation Process
Pilot Findings: What Did We Learn?
Data Heterogeneity: A Persistent Challenge in Pharmacy Systems
While a plethora of federal regulations have slowly pushed electronic health record systems in physician and hospital settings towards standardization, pharmacy data systems remain highly variable in what data they capture, how the data is captured and what is available for use in standardized measurement.
Throughout the pilot, each SP organization demonstrated how the structure of their own specific systems, configurations and data element terminologies affected measure calculations. Critical data elements required to calculate the measure are not yet standardized across participating SP systems, highlighting how differences in data definitions can impact the generalizability of measure rates. Notably, many organizations had to combine several fields within their existing data to derive measure specific data elements, such as profiled time, which can make direct comparisons of measure rates challenging. However, some participants were able to incorporate technology and workflow improvements to allow for the capture of more consistent and precise data needed to calculate TAT.
Despite the challenging nature of the data, participants universally agreed that it is beneficial to standardize the way SPs measure TAT across the industry.
Barriers, Facilitators and Promising Practices for Measure Use and Improving TAT
Throughout the pilot, PQA gathered feedback from participants on barriers, facilitators and promising practices to improve TAT. This feedback was gathered via surveys and then further discussed by pilot participants during three collaborative webinars. Key themes from those discussions include:
Facilitators for Improving TAT
- Staffing levels emerged as an important theme. Sufficient staffing enabled specialty pharmacies to take on new tasks (e.g., calculating TAT) and distribute the workload amongst employees and allow staff to have dedicated time to address prescription processing or patient needs (e.g., prior authorization), which affect TAT.
- One pilot participant noted that during the COVID-19 pandemic, their staffing was reduced by more than a third compared to their normal operating levels. As staffing levels continued to normalize to pre-pandemic levels, participants noted a reduction in TAT.
- Technology improvements can facilitate TAT by streamlining processes for quicker triage of incoming prescriptions. Information systems improvements can also allow greater visibility into data and enable more timely interventions for prescriptions that require additional processes, such as prior authorization. Additionally, identifying key activities that qualify for automation can reduce redundant processes, freeing up resources to focus on improvement efforts.
- Improved communications between pharmacists and technicians leads to more efficient TAT. For example, one participant described how varying levels of “urgency” could be communicated to pharmacists, empowering them to prioritize prescriptions for expedited review. Additionally, improving communication between providers and pharmacists can enable quicker resolution for prescriptions that cannot be dispensed or scheduled for delivery due to interventions needed to address cost, medication availability, or medication interactions identified by the pharmacist.
Barriers to Optimizing TAT
-
Prior authorizations are often responsible for longer TAT, with many participants expressing the burden of rigorous documentation required by payers. Having processes in place to identify and triage prescriptions requiring prior authorization efficiently can help minimize delays.
-
Differring patient needs or preferences require different coordinated approaches. Participants noted that not all forms of outreach are equally effective for different patients; there is no “one-size-fits-all” intervention. For example, some patients are more technology-averse and can have longer TAT compared to populations that have embraced electronic communications. “Meeting patients where they are” can help to improve communications and ultimately reduce TAT.
-
Navigating patient assistance programs can affect TAT as specialty pharmacies work through benefit challenges with payers and manufacturers. Not only do specialty pharmacies need to complete initial prior authorizations, but specialty medication fulfillment can be further complicated by overrides and the need to clarify benefits with insurance companies, patients, manufacturers and other parties. This complex coordination can result in delays.
-
Broader education for pharmacists, as well as streamlined and better coordinated benefit investigations, has the potential to alleviate some of the burden associated with this complicated area.
-
Medication shortages can impact TAT, with shortages leading to longer TATs for certain types of medications. Participants also noted that they sometimes experienced delays in receiving generic inventory and had to revert to branded products in certain scenarios. These dynamics can also lead to longer TATs.
Measure Performance: Clusters with Variability
Baseline, midpoint and endpoint SP-TAT-PH measure rates varied across participants, with a pair of observed clusters. Between baseline and endpoint, SPs saw both increases and decreases in TAT, which were generally small in magnitude. Full pilot results, including performance rates and interpretations, will be available in future publications.
Want to Learn More?
Save the date for a Quality Essentials webinar on September 19, from 1:00-2:00 p.m. ET, to learn more about the PQA Specialty Pharmacy Turnaround Time [Pharmacy] quality measure and this pilot, including insights from the pilot participants! Registration information will be shared later this summer.
Join us in person at the NASP 2024 Annual Meeting & Expo in Nashville, Tenn., on October 8, at 4:30 p.m. CT, for our session titled “Piloting a Specialty Pharmacy Turnaround Time Quality Measure: Findings and Lessons Learned.”
