 PQA uses a systematic, transparent, and consensus-based process to conceptualize, specify, test, and endorse quality measures. Following endorsement by PQA’s membership, PQA measures can be implemented into various quality programs, including those maintained by the Centers for Medicare & Medicaid Services (CMS). Many PQA measures are used in CMS Quality Programs to evaluate safe and appropriate medication use among beneficiaries.
PQA uses a systematic, transparent, and consensus-based process to conceptualize, specify, test, and endorse quality measures. Following endorsement by PQA’s membership, PQA measures can be implemented into various quality programs, including those maintained by the Centers for Medicare & Medicaid Services (CMS). Many PQA measures are used in CMS Quality Programs to evaluate safe and appropriate medication use among beneficiaries.
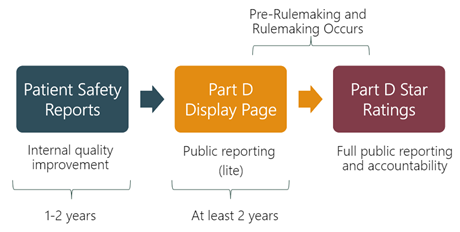
CMS created the Medicare Part D Star Ratings to provide quality and performance information to Medicare beneficiaries to assist them in choosing their prescription drug services. The Part D Star Rating for each Medicare Advantage (MA-PD) or standalone prescription drug plan (PDP) is determined through performance on numerous measures across several domains. Each measure is awarded a star rating and the individual measure stars are then aggregated at the domain and summary level. Five PQA measures are currently implemented in the Part D Star Ratings:
- Medication Adherence for Diabetes Medications
- Medication Adherence for Hypertension (RAS antagonists)
- Medication Adherence for Cholesterol (Statins)
- MTM Program Completion Rate for CMR
- Statin Use in Persons with Diabetes
CMS also reports performance on quality measures for Part D plans through its Display Page and Patient Safety Reports. CMS publicly reports plans’ performance on display measures through the Part D Display Page. After at least two years on the Display Page, measures can be considered for inclusion in the Star Ratings through CMS’ pre-rulemaking and subsequent rulemaking processes. For 2023, seven PQA measures will be included on the Part D Display Page:
- Initial Opioid Prescribing for Long Duration (IOP-LD) (NQF #0558)
- Antipsychotic Use in Persons with Dementia (APD)
- Concurrent Use of Opioids and Benzodiazepines (COB) (NQF #3389)
- Use of Opioids at High Dosage in Persons Without Cancer (OHD) (NQF #2940)
- Use of Opioids from Multiple Providers in Persons Without Cancer (OMP) (NQF #2950)
- Polypharmacy: Use of Multiple Anticholinergic Medications in Older Adults (POLY-ACH)
- Polypharmacy: Use of Multiple Central Nervous System-Active Medications in Older Adults (POLY-CNS)
The Patient Safety Reports provide plans with performance data intended for internal quality improvement, and are not available to the public. All PQA Star Ratings measures and Display measures are also reported in the Patient Safety Reports, except for MTM Program Completion Rate for CMR. As of 2023, two PQA measures are included exclusively on the Part D Patient Safety Reports:
- Medication Adherence for HIV/AIDS (Antiretrovirals) (ADH-ARV)
- Persistence to Basal Insulin (PST-INS)
 Beyond Medicare Part D, PQA measures are also used to evaluate performance in CMS’ Medicaid Adult Core Set as well as the Health Insurance Marketplace Quality Rating System (QRS).
Beyond Medicare Part D, PQA measures are also used to evaluate performance in CMS’ Medicaid Adult Core Set as well as the Health Insurance Marketplace Quality Rating System (QRS).
The Medicaid Adult Core Set program aims to publish and track state-level quality measures for adult Medicaid beneficiaries and estimate national quality of care for Medicaid beneficiaries. Annual ratings are reported on Medicaid.gov for measures with at least 25 states reporting. The 2023 and 2024 Adult Core Set consists of 34 measures across six domains. The Department of Health and Human Services requires review and update of core sets on an annual basis. CMS convenes a multistakeholder workgroup to review existing measure sets and evaluate measure additions and removals based on technical feasibility, strategic priority, and other considerations.
For 2023, two PQA measures are included in the Medicaid Adult Core Set and fall under the domain of Care of Acute and Chronic Conditions:
- Use of Opioids at High Dosage in Persons Without Cancer (OHD-AD)
- Concurrent Use of Opioids and Benzodiazepines (COB-AD)
Lastly, the Health Insurance Marketplace QRS aims to help consumers make informed decisions about health insurance coverage, facilitate oversight of qualified health plans, and provide actionable information to health plans to improve the quality of services they provide. Annual quality ratings are displayed on healthcare.gov when consumers view the list of qualified health plans available in their area. Performance data is released at CMS.gov via a Public Use File (PUF).
Five PQA measures are included in the 2023 QRS Measure Set:
- Proportion of Days Covered: Diabetes All Class (PDC-DR)
- Proportion of Days Covered: Renin Angiotensin System Antagonists (PDC-RASA)
- Proportion of Days Covered: Statins (PDC-STA)
- Annual Monitoring for Persons on Long-Term Opioid Therapy (OHD)
- International Normalized Ratio for Individuals on Warfarin (OMP)
The use of selected PQA measures across multiple CMS programs is summarized below.
| Selected PQA Measures | Medicare Part D | Medicaid Adult Core Set | Health Insurance Marketplace QRS |
| Proportion of Days Covered: Diabetes (PDC-DR) | X | X | |
| Proportion of Days Covered: RASA (PDC-RASA) | X | X | |
| Proportion of Days Covered: Statins (PDC-STA) | X | X | |
| Use of Opioids at High Dosage in Persons Without Cancer (OHD) |
X | X | |
| Use of Opioids from Multiple Providers in Persons Without Cancer (OMP) | X | ||
| Concurrent Use of Opioids and Benzodiazepines (COB) | X | X | |
| International Normalized Ratio for Individuals on Warfarin (INR) | X | ||
| Annual Monitoring for Persons on Long-Term Opioid Therapy (AMO) | X |
